mRNA
High quality mRNA at high yields
mRNA is a promising and rapidly growing new type of advanced medicine with quick production speed, the ability to encode varied proteins, an excellent safety and efficacy profile, and flexibility in modality and formulation. We are able to provide end-to-end CRO (click here for mRNA CRO service) and CDMO services. Based on the well-established and high-efficient mRNA platform, we continue to acceralate your products from clinics to beyond.
At Porton Advanced, we have established an expert mRNA manufacturing process, including USP mRNA production (IVT, enzymatic/co-transcriptional capping poly(A) tailing, etc. with yield up to 10mg/mL), DSP mRNA purification (affinity chromatography, ion-exchange polishing, TFF, etc.) and lipid nanoparticle (LNP) delivery formulation. The validated process can greatly reduce the project timeline and deliver results with robust quality and high efficiency.
Our Services
We are a trusted partner to our clients, providing end-to-end services from plasmid process development to GMP mRNA manufacturing:
mRNA Process Development
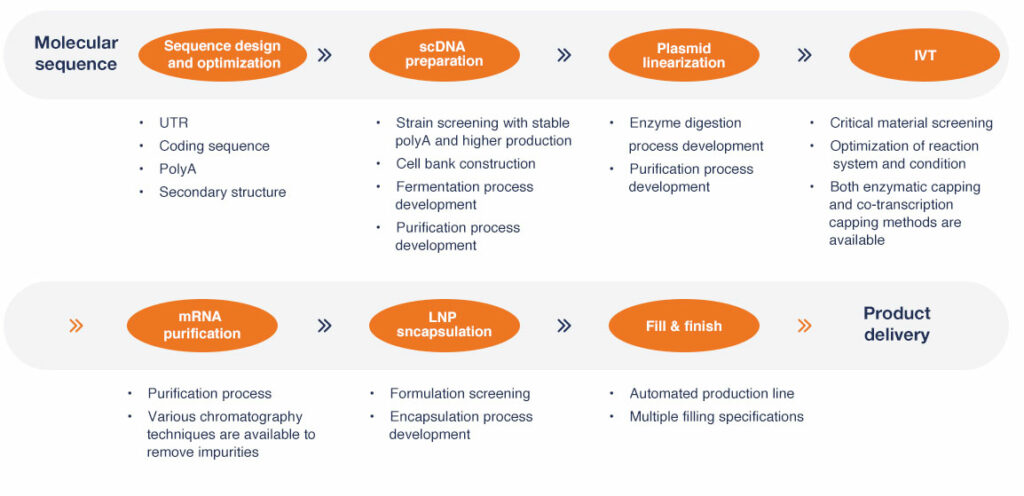

cGMP mRNA PRODUCTION
Production work flow
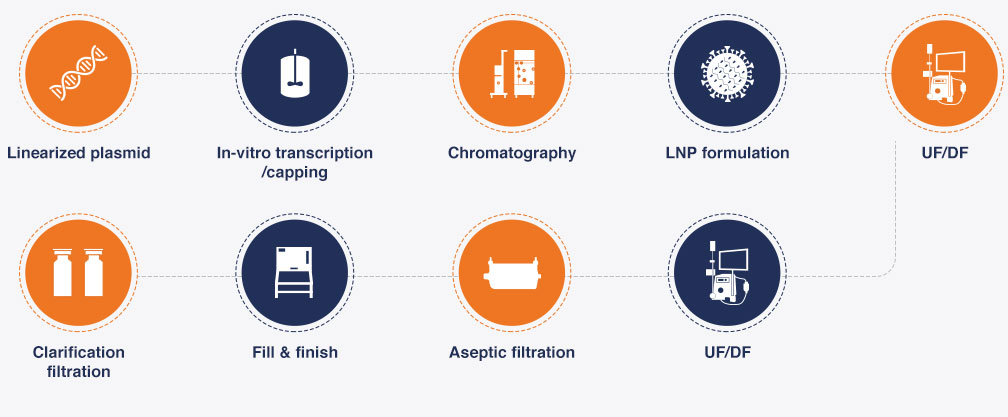
Production Process Flow Diagram for mRNA and circRNA products
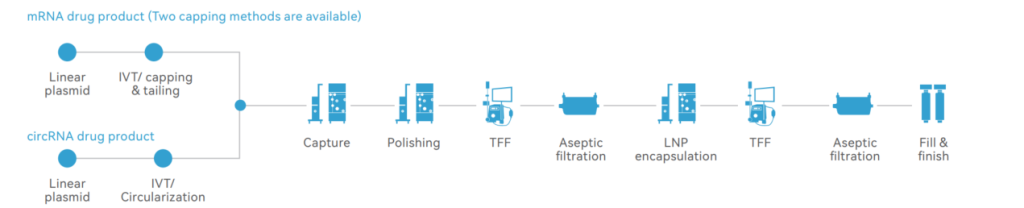
Process Platforms Dedicated to Different Types of RNA
Porton Advanced has established numerous types of mRNA-related synthesis, capping, and purification processes. The robust process enables fast and guaranteed delivery.
Microbial strain and plasmid template for mRNA
- Clear traceability and reduced license fee compared with other commercial strains
- Solving issues about plasmid polymerization and poly(A) tails shortening
- Knock out genes related to recombination and repairing
- Supercoiled ratio ≥ 95%
- No antibiotic resistance gene
Capping methods for non-replicating and self-amplifying mRNA
- Two capping methods include co-transcription capping and enzymatic capping.
Circulation and purification methods for circular RNA
- Two circulation methods include the enzymatic circularization method and group I Intron circularization.
- Two purification methods include RNase R-assisted purification and affinity chromatography.
We are partnering with GeenSeed Biotech to propel technological innovation and clinical translation jointly, thereby accelerating the implementation of innovative circRNA therapies.
Related News:https://portonadvanced.com/latest-news/porton-advanced-and-geneseed-biotech-enter-into-strategic-collaboration-to-focus-on-advancing-circrna-innovative-therapeutics/
LNP Delivery System from Porton Advanced’s Strategic Partners
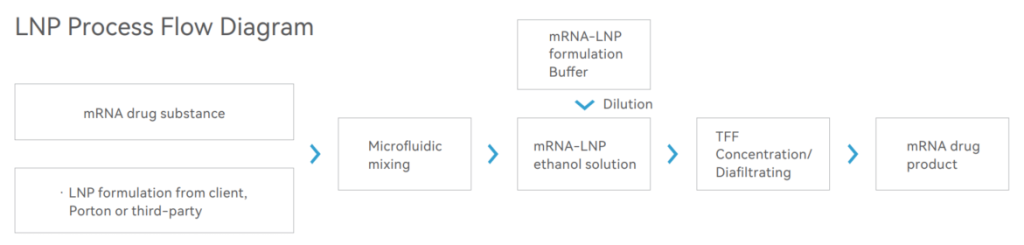
Currently, we are partnering with some of the most promising mRNA R&D companies in China, which have extensive knowledge of mRNA Technology and believe they have leading mRNA-LNP technology platforms. They are actively pursuing exploring the best-in-class LNPs in diverse application areas including infectious vaccines, cancer vaccines, liver delivery, immune cell delivery, and local delivery. We could use LNP formulations either from clients or our partners for subsequent encapsulation and fill & finish steps.
GMP Fill&Finish System
We can provide GMP fill & finish systems for both mRNA drug substance and drug production. For mRNA drug substance, the filling scale is around 3000 vials per hour and 12000 vials per batch and the system accommodates 2R/6R/10R/20R.

mRNA Case studies
- Integrity Test of mRNA (CGE by PA800)
- mRNA Capping Ratio by LC-MS
- mRNA-LNP key testing shows stable quality with minimal lot-to-lot.
- LNP-circRNA transfection efficiency and biocompatibility
Porton Advanced’s Analytical platform utilized the CGE method for assessing mRNA integrity. Results showed a high level of mRNA intactness, meeting the process development standard.
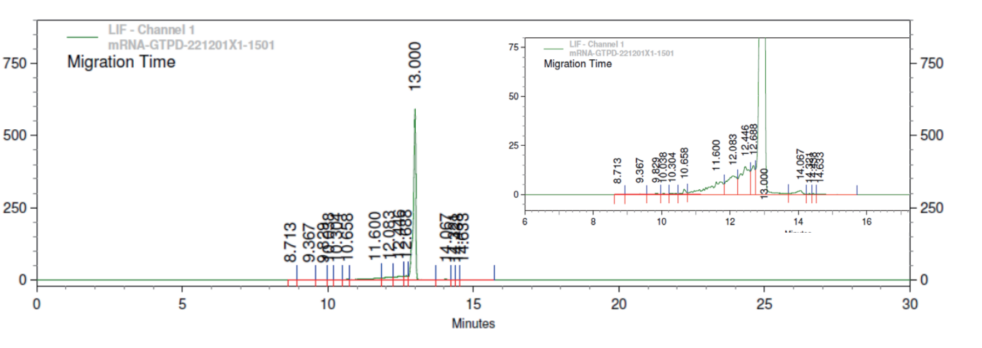 (source: Porton Advanced’s Analytical Development Platform)
(source: Porton Advanced’s Analytical Development Platform)
Porton Advanced’s analytical platform performed capping ratio analysis on IVT mRNA using LC-MA. The capping rate of samples developed by the platform process is over 98%.
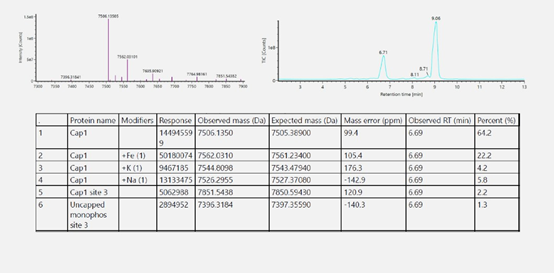
(source: Porton Advanced’s Analytical Development Platform
Porton Advanced’s analytical platform showed that particle size and particle distribution present stable quality with minimal lot-to-lot. Other key tests show that encapsulation efficiency and mRNA integrity are over 90%.
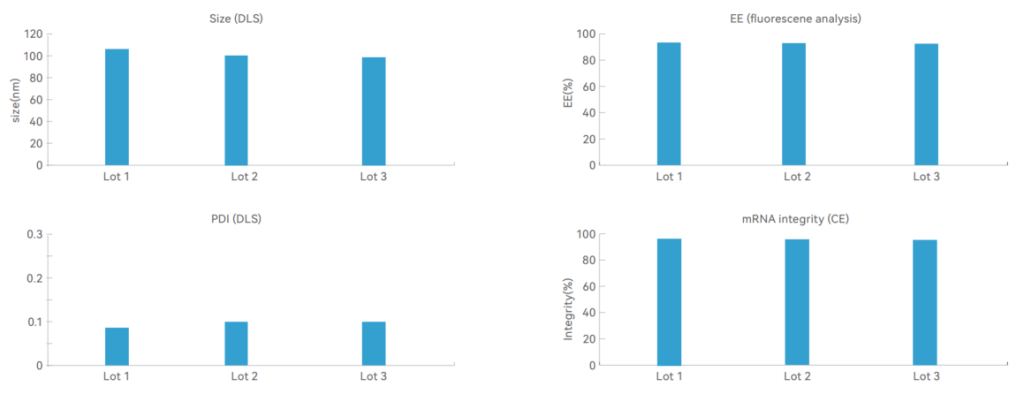
(source: Porton Advanced’s Analytical Development Platform)
Compared to Lipo8000, LNP-circRNA showed higher transfection efficiency and biocompatibility. Through different routes of administration (intramuscular injection and intravenous injection), LNP-circRNA shows consistency in transfection efficiency in mice.
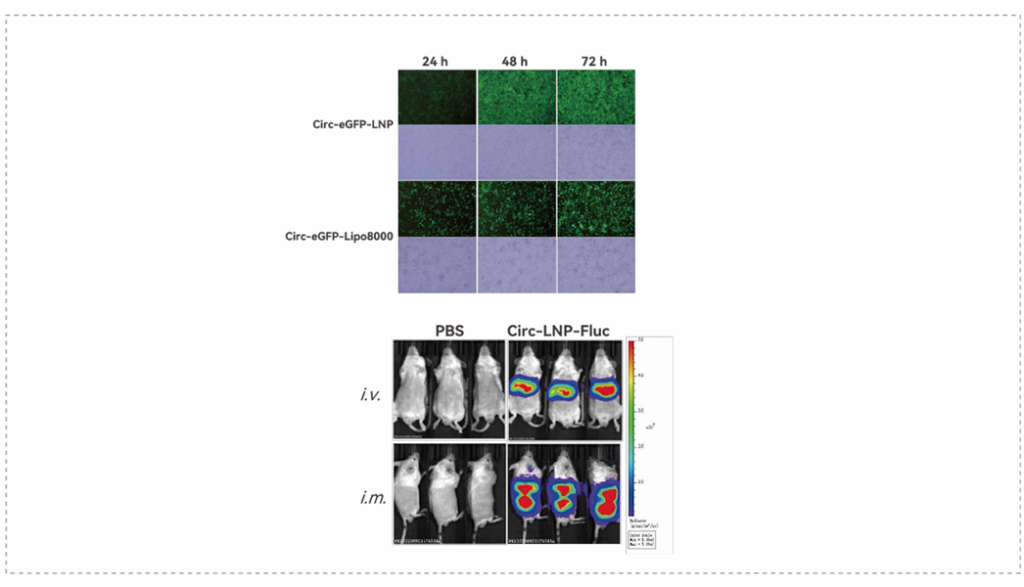
(source: Porton Advanced’s Analytical Development Platform)
WHY CHOOSE PORTON ADVANCED’S MRNA CDMO PLATFORM?
Robust Process Enables Fast Delivery
- Only 10 weeks to deliver mRNA drug products for IIT
- Only 4 months to deliver mRNA-LNP engineering run
Experienced in IND application and more
- Obtained multiple global IND approvals (one FDA and one MedSAFE)
- Multiple going-on mRNA IND projects
For more specific:
End-to-End CDMO Services:
- From sequence design to GMP LNP encapsulation
- Fill & Finish for mRNA drug substance and drug products (over 10,000 vials/batch)
Microbial Strains and Plasmid Templates Dedicated for mRNA
- Cost-saving compared to other commercial strains
- Solving issues for plasmid polymerization/poly(A) tails shortening
Diverse Process Methods Accommodating Different RNA Types
- Non-replicating, self-amplifying, and circular RNA
- Capping methods for mRNA
- Circularization methods for circular RNA
- Chromatography and purification methods for plasmids
Third-party LNP Formulations available
- LNP formulations from Porton Advanced’s strategic partners: LNPs targeted for vaccines, liver, immune cells, and other organs.